Guidelines and standards of good manufacturing practice
Avoid manufacturing defects by indicating your good manufacturing practice, checks and processes to the operator.
Create a GMP for each recipe
Register a guide for each reference you produce. Establish the progressive stages to be completed and indicate and groups all fields, required or optional, such as checks, machine parameters, weight records or batch allocation.
Release a standard of good manufacturing practice to enable its use in the preparation or mixing room. Only qualified personnel may release and sign a guide. Version control provides the most current version of each recipe or preparation.
Assign guides to processes, machines or users
We can assign a specific guide to different sections, machines, articles, and processes, or indicate that they should be started every certain number of units manufactured, minutes or hours.
An automatic prompt on the operator’s terminal will indicate to complete the steps in the guide at the indicated time.
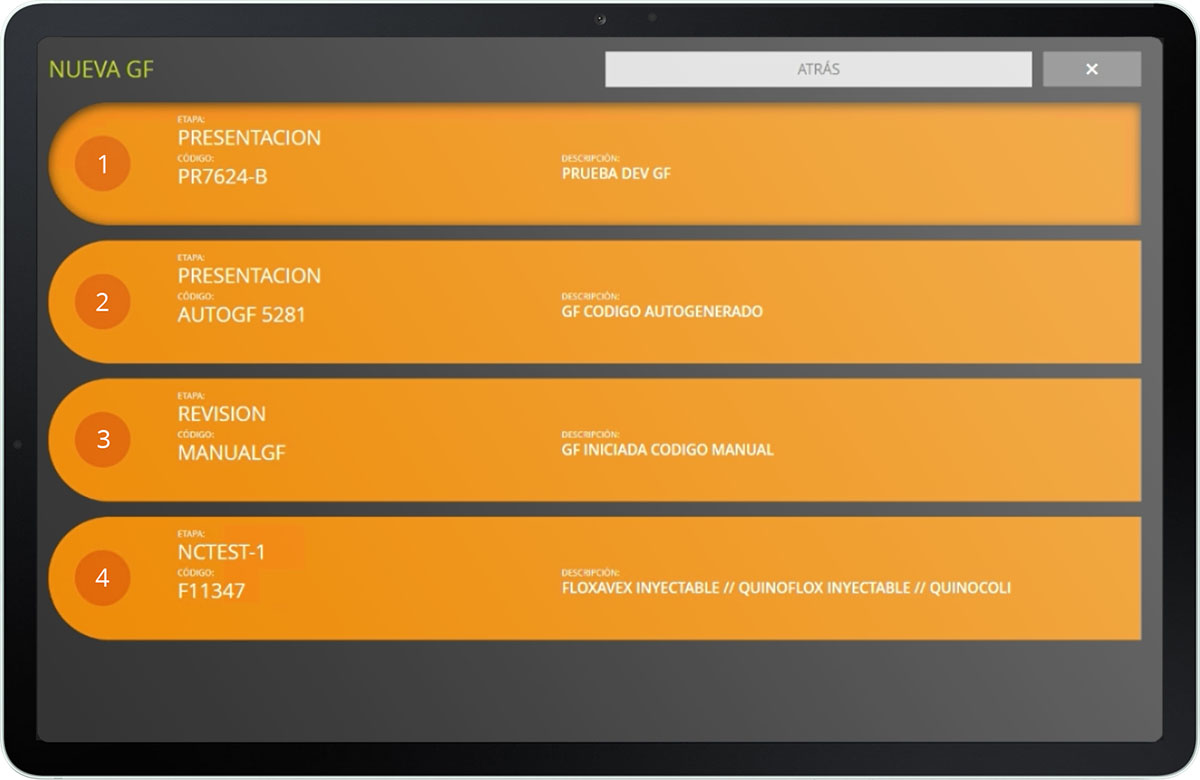
Verify compliance with good manufacturing standards
Check if the steps of each GMP have been completed and validate the imputed records in each production order.
Check through the reports generated the date on which the guide was activated, who has performed the controls and their level of compliance.
An additional layer of quality
Establish the stages of the manufacturing guide for each item or reference so that a specific quality control form is activated in each of them.
Manufacturing guides are a layer of control to avoid errors in the preparation of recipes and semi-finished products.
Warn the operator if he has pending steps
If any step of the guide is not completed, doeet highlights a warning on the terminal with the actions still to be performed.
If the steps are mandatory for the recipe, the system does not allow you to finish the guide without entering the relevant data.

Advantages of Good Manufacturing Guidelines and Standards
Create the recipes with the steps to follow for each item.
Control every step of your production processes and recipes.
Manage the roles and users involved in each recipe.
Avoid traceability errors in raw materials and semi-finished products.
Review the compliance of each stage with its associated records.
Functions of the guides and standards of good manufacturing practice

- Structured monitoring and control of all the factory’s production processes.
- Definition and assignment of recipes and manufacturing guides and their stages to each reference.
- Generation of the controls to be completed by the operator.
- Fulfilment of the stages in the terminal at the plant.
- Validation of the controls by the plant manager and/or quality manager.
Other doeet solutions for Warehouse and Traceability